Antipyretics for children are prescribed by a pediatrician. But there are situations of emergency care for fever, when the child needs to give the medicine immediately. Then the parents take responsibility and apply antipyretic drugs. What is allowed to give to infants? How can you bring down the temperature in older children? Which medications are the safest?
Many of us know that you can not directly connect copper and aluminum wires. There are several answers to this question.
Myth number 1. Aluminum and copper have a different coefficient of thermal expansion. When current passes through them, they expand differently, when the current stops, they cool differently. As a result, a series of extensions-contractions changes the geometry of the conductors, and the contact becomes loose. And further on, in the place of bad contact, heat occurs, it deteriorates even more, an electric arc appears, which completes the whole thing.
This opinion is untenable because the linear coefficient of thermal expansion for metals used for electrical installation: copper - 16.6 * 10-6m / (m * g. Celsius); aluminum - 22.2 * 10-6m / (m * gr. Celsius); Steel - 10,8 * 10-6m / (m * gr. Celsius). However, the differences in linear thermal expansion are relatively easily compensated by the use of reliable clamps that create a constant pressure on the contact. Expand the metals, compressed with a well-tightened bolted joint, remains only to the side, and temperature drops are not able to seriously weaken the contact.
Myth number 2. Aluminum forms on its surface an oxide non-conductive film, which from the very beginning worsens the contact, and then the process goes on the same increasing: heating, further deterioration of contact, arc and destruction. This option is also not entirely correct, since the oxide film allows to connect aluminum conductors with steel and with other aluminum conductors.
Myth number 3. Aluminum and copper form a "galvanic pair", which simply can not be overheated at the point of contact. And again heating, arc and so on. However, the copper conductor is also quickly covered with oxide, with the only difference being that copper oxide conducts more or less current. If a copper and aluminum conductor are connected, their oxides have the possibility of decay into charged ions. The ions of aluminum oxide and copper, being particles with different electrical potential, begin to take part in the process of current flow. A process known as "electrolysis" begins. In the course of electrolysis ions transfer charges and move themselves. When they move, the metal collapses, shells and voids are formed. Especially it concerns aluminum. Well, where there are voids and shells, there is no longer any reliable electrical contact. Bad contact starts to get warm, gets worse and so on until the fire.
Especially dangerous is the connection of copper and aluminum wires in the street. Under the influence of natural moisture and passage through the connection of electric current, the process of electrolysis takes place and on the street the process of contact destruction is significantly accelerated. As a result, conchs form at the junction, heating and sparking of contacts occur, charring of insulation.
How to properly connect copper and aluminum conductors.What to do when you combine different metals is really necessary? There are only two ways left: to connect through another metal or to eliminate the formation of a destructive oxide film. In the first case, a variety of connectors are used: terminal blocks without direct contact of dissimilar conductors, a protective layer of the third metal, washers, special tips.

For joining copper and aluminum, special pastes are used, which protect the contact from oxidation and moisture penetration, and prevent the subsequent destruction of the contact. If you need a third for the friendship of these two metals, then one of them can be zaludit. For example, a tinned copper multicore wire will perfectly perform the task at connection with a single-core aluminum.

For a specific task of connecting to an aluminum riser in an access panel, tapping clamps (pinches) with or without punctures are used. They have an intermediate plate that excludes direct contact. There are copies with or without paste. For more common tasks it is possible to use terminal blocks with partitions or different sockets for conductors made of copper and aluminum. You can even use the usual bolted connection, the main thing is not to forget to lay a washer between the copper and aluminum wires, galvanized or stainless steel.
Repairing electrical wiring in old houses, you can face a situation where you have to change large sections of the wiring. However, in most cases, the old wiring is made of aluminum, and for replacement only a copper wire is available. In general, it is strictly forbidden to connect conductors from such different materials, but it happens that there is simply no other way out. Consider how to still connect the aluminum and copper wires so that there is no short circuit or fire.
For this, it is worth to strain your memory and remember the school course of chemistry and physics.
To begin with, let us recall what is galvanic cell. Simply put, a galvanic cell is a simple battery that generates an electric current. The principle of its appearance is based on the interaction of two metals in the electrolyte. So, the twist between the copper and aluminum wire and will be the same battery.
Galvanic currents quickly destroy the material. True, in dry air their appearance is excluded. And if you twist the rosette, it will not fall apart in a few hours. However, afterwards troubles to such wiring are ensured.
Over time, the materials from which the wires are made are destroyed, along with this constantly resistance increases. If a power consumer is connected to the outlet, the twist will begin to heat up. With the regular use of such a socket, the threat of a fire increases.
Therefore, it is forbidden to connect an aluminum conductor with a copper wire. However, there are emergency situations when making such a connection is simply necessary.
Let's consider several ways how to connect an aluminum and copper wire. These methods will help successfully cope with the difficult business.
Scrolling
Is an the simplest way Mount the wires. It does not require special knowledge and skills. However, it is not the most reliable way to connect. Due to temperature fluctuations, the metal expands. As a result, a gap is formed between the conductors, increasing the resistance. After some time, the contact oxidizes and breaks down.
Of course, this will not happen within a year, but if the connection should be profounded for a long time, then it is worthwhile to think about other ways of bonding.
The very principle of fastening by the twisting method is that both conductors twisted each other. For a better connection, the copper cable is brazed with solder. Stranded copper wire will have to be compulsorily compulsory.
Threaded connection
To join copper and aluminum in this way, a pair of simple washers, one spring washer, a screw and a nut. This method is very reliable - contact between conductors will be provided for many years. For this fastening, neither the cross-section of the wire nor its type is multi-stranded or solid.
The insulation is removed from the end of the wire. The spring washer is put on the screw, then the usual washer is put on, then the ring of the aluminum wire. It is supported by a simple washer. Then the copper conductor is put on, and then the nut is screwed onto the screw. She tightly squeezes the entire joint.
The multicore cable must be welded with solder before the connection.
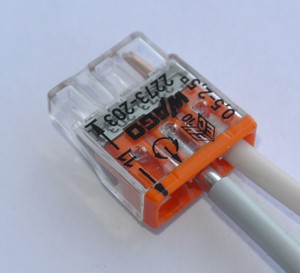
Connection using the terminal block
This is a modern method of mounting wires. Although it loses a bit of reliability in the threaded connection method , the method has its advantages:
- the connection can be made very quickly;
- when connecting you can do with a small supply of wire.
The last to explain, it happens that a small piece of cable sticks out from the wall or ceiling. It is impossible to twist the wires - very few wires. Yes, and made on the ceiling twist will not last long, after some time the wires just break off. And the terminal block will hold both conductors for a long time. Then the shoe completely eliminates the contact of two stripped conductors.
The installation is carried out as follows: the bare end of the wire (about 5 mm.) Is inserted into the terminal hole of the pad, after which the locking screw is tightened.
The terminal block can not be hidden in plaster or in a wall without a junction box.
Flat-spring clamp and terminal block
This method appeared not so long ago. There are two types of this connection: one-time and one-time. For the last connection there is a special lever in the terminal block. Thanks to him, the wire can be inserted and removed several times. Terminal blocks of this type can successfully connect copper and aluminum multicore wires of various types.
Widely used for installation of chandeliers and wiring connections in junction boxes . It takes some effort to insert the wire into the hole in the terminal block. To pull out the conductor you will need to apply even more effort. For practical use it is better to use reusable models. In case of an error, the connection can be quickly changed.
It is very simple to perform such installation. First off the cable removes insulation (approximately 10 mm.). Then on the reusable terminal block you need to raise the lever, insert the wire, and then return the lever to its original position. It's simple!
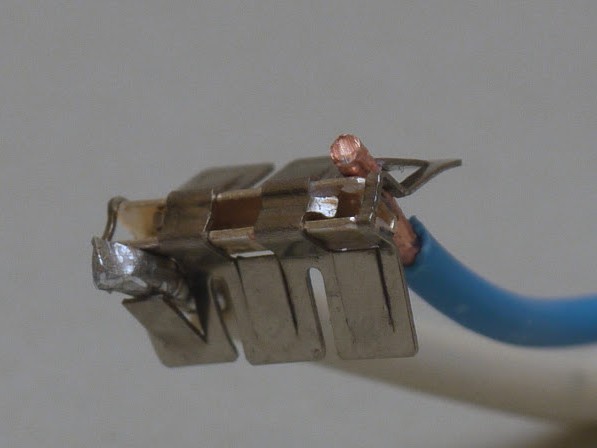
Rivet
Reliability is not inferior to a threaded joint and has its own advantages and disadvantages:
- this connection is established very quickly;
- it is very durable, reliable and affordable;
- however, unlike threaded fasteners, this connection is one-time.
Mounting is performed using a special tool - rivet. An aluminum wire is put on the rivet, then a spring nut, followed by a copper wire and a flat washer. Then the rivet is in use and the connection is ready.
It is worth mentioning that the connection site must be isolated.
Soldering
Is it possible to solder conductors made of different materials? It is quite possible, if meet certain conditions.
With copper soldering problems will not arise, unlike aluminum. On the surface of this metal, an amalgam is formed, exhibiting remarkable chemical resistance. That is, the solder can not stick to it. This phenomenon often causes surprise for novice electricians.
To solder two different conductors should be stocked with a solution of copper sulfate, a "Crohn" battery and a piece of copper wire. On the aluminum wire neatly cleaned the future place of soldering. Then the place is dripped solution of copper sulfate.
The copper wire is connected to the positive pole of the "Krona" battery and lowered into copper sulfate. An aluminum conductor is connected to the negative terminal of the battery. After a while, a layer of copper settles on the aluminum, which can be soldered to the required wire without any problems.
Conclusion
Once again, it should be noted that any connection of the wires must be insulated.
You can place connections in special junction boxes.
If the connection is planned to be made with your own hands, then there is no need to resort to the soldering method. It requires certain experience and qualifications. It is better to use another of the above methods of connecting aluminum and copper conductors.
The most accessible and common methods were considered in the article. However, in the absence of experience in conducting such work, it is better to turn to professionals.
Often even in the case of pulling a new wiring, you have to connect the copper wires to the aluminum wires. Yes, even at the entrance to the house, because the supply line of electricity from aluminum, and therefore connect it should also be an aluminum wire or copper, but with reservations. It is impossible to connect these two metals directly, and that's why it happens. Copper and aluminum are metals of different activities, they have different resistances, and their physical properties are different. For copper, the current moves with the least resistance, which means that the transmission capacity of copper wires is higher. Not only that, but in the case of direct twisting of copper and aluminum wires, problems arise.
What happens with straight twisting
First, we'll figure it out with bandwidth. Imagine that you are letting water through a pipe of arbitrary diameter. Let's gradually increase the water pressure. Sooner or later there will come a time when the capacity of the pipe is not enough, the pressure in it will begin to increase and it will burst. Almost the same happens in the wire. Increased resistance in aluminum will cause it to warm up if it is twisted with a copper wire of the same cross section. But the most important thing happens exactly in the place of twist.
Chemical characteristics of metals
Reacting with oxygen of air and moisture, metals, as is known, begin to oxidize. The rate of oxidation and the properties of the oxide film are different for them. In the case of copper, this process proceeds slowly enough, and the oxide film has a good current conductivity. But on aluminum, the oxide film appears many times faster, and it conducts the current very badly. As a result, a zone of increased or active transient resistance is created on the twist, almost like in a spiral of your home electric kettle or iron. Heavy heating occurs. But that is not all.
Some physical properties of metals
Also, everyone is well aware of the linear expansion of metals. In copper and aluminum they are different. Given the load - the twisting has heated up, the wires widened unevenly, lifted the load - narrowing occurred, twisting weakened. Very quickly the density of twisting is lost - it starts to spark! This is the most dangerous moment when high temperatures combined with sparking cause a fire.
How to avoid problems?
A few simple rules:
- Address to professionals, ordering services of electroassembly - they will do everything right, even if you need to connect copper and aluminum wires
- Use transition metals or special connectors - an ordinary metal bolt, three washers and a nut - here's a primitive way of connecting through metal. But in the market of electrical equipment the mass of various connectors on the terminals, which are specially designed for this, there are also transition plates
- Tinning - if at hand only a soldering iron and solder - go ahead, peel the copper wire (with an aluminum wire it will not work, and already it will not be necessary)
- Lubricants - additionally use special lubricants that do not allow metals to oxidize
- Correctly calculate loads - In any case, the conductor of the aluminum wire should be of a larger cross section than the copper wire. Otherwise, the aluminum section will warm up
You can buy all the special connectors and lubricants in electrical equipment stores, but they always have specialists. And the last tip - do not save. Let all the wiring be better from copper wires, although this will cost more. But you will do once and forget about the problems. Moreover, companies that provide wiring services, offer materials at the most favorable prices, which you will not see in stores.
What is in electrical engineering can not directly connect copper and aluminum conductors, is not a secret even for many ordinary people who have nothing to do with the electrician. On the part of the same townsfolk, electricians often ask the question: "Why?"
Why any age can stall anyone. Here is a similar case. A typical professional answer: "Why, why ... Because it will burn. Especially if the current is large. " But this does not always help. As after this often follows another question: "And why will it burn? Why does copper with steel not burn, aluminum with steel does not burn, and aluminum with copper burns? "
On the last question you can hear different answers. Here are some of them:
1) In aluminum and copper, a different coefficient of thermal expansion. When current passes through them, they expand differently, when the current stops, they cool differently. As a result, a series of extensions-contractions changes the geometry of the conductors, and the contact becomes loose. And further on in the place there is heating, it worsens even more, there is an electric arc, which completes the whole thing.
2) Aluminum forms on its surface an oxide non-conductive film, which from the very beginning worsens the contact, and then the process goes on the same increasing: heating, further deterioration of contact, arc and destruction.
3) Aluminum and copper form a "galvanic pair", which simply can not not be overheated at the point of contact. And again heating, arc and so on.
Where is the truth, in the end? What happens there, at the junction of copper and aluminum?
The first of these answers is still untenable. Here are tabulated data on the linear coefficient of thermal expansion for metals used for electrical wiring: copper - 16,6 * 10-6m / (m * gr. Celsius); aluminum - 22.2 * 10-6m / (m * gr. Celsius); Steel - 10,8 * 10-6m / (m * gr. Celsius).
Obviously, if the matter were in the expansion coefficients, the most unreliable contact would be between the steel and aluminum conductor, because their expansion coefficients differ by a factor of two.
But even without tabular data it is clear that the differences in linear thermal expansion are relatively easily compensated by the use of reliable clamps that create a constant pressure on the contact. Expand the metals, compressed, for example, with a well-tightened bolted joint, remains only to the side, and temperature changes can not seriously weaken the contact.
The option with the oxide film is also not quite true. After all, this same oxide film allows you to connect aluminum conductors with steel and with other aluminum conductors. Yes, of course, the use of a special lubricant against oxides is recommended, yes, a systematic revision of compounds involving aluminum is recommended. But all this is allowed and works for years.
But the version with a galvanic pair really has a right to exist. But here, after all, it does not do without oxides. After all, the copper conductor is also quickly covered with oxide, with the only difference that copper oxide conducts more or less current.
In the course of electrolysis ions transfer charges and move themselves. But, in addition, ions are after all metal particles of conductors. When they move, the metal collapses, shells and voids are formed. Especially it concerns aluminum. Well, where there are voids and shells, there is no longer any reliable electrical contact. Bad contact starts to get warm, gets worse and so on until the fire.
It should be noted that the more humid the ambient air, the more intensively all the listed processes proceed. A non-uniform thermal expansion and a non-conductive layer of aluminum oxide are only aggravating factors, nothing more.
About that that in electroconducting can not connect copper and aluminum wires, even many townsfolk know, not to mention professional electricians. In this article, we will try to answer the question: "Why this can not be done?". It would seem that, according to the old rules and regulations, both copper and aluminum wires were used in electric wiring. They could exist freely even in one wiring! There could be (separate lines), but there is no connection! If they were connected, then you had to constantly monitor the connection points. Otherwise, heat and fire!
On this occasion, a natural question arises: " And why is there a fire? Why does copper with steel do not burn, aluminum with steel does not burn, and aluminum with copper burns ?! "
On the last question you can hear different answers. Here are some of them:
Aluminum and copper have a different coefficient of thermal expansion. When current passes through them, they expand differently, when the current stops, they cool differently. As a result, a series of extensions-contractions changes the geometry of the conductors, and the contact becomes loose. And further on, in the place of bad contact, heat occurs, it deteriorates even more, an electric arc appears, which completes the whole thing.
Aluminum forms on its surface an oxide non-conductive film, which from the very beginning worsens the contact, and then the process goes on the same increasing: heating, further deterioration of contact, arc and destruction.
Aluminum and copper form a "galvanic pair", which simply can not be overheated at the point of contact. And again heating, arc and so on.
Where is the truth and what is really happening at the junctions of copper and aluminum?
The first of these answers is still untenable. Here are tabulated data on the linear coefficient of thermal expansion for metals used for electrical wiring: copper - 16,6 * 10-6m / (m * gr. Celsius); aluminum - 22.2 * 10-6m / (m * gr. Celsius); Steel - 10,8 * 10-6m / (m * gr. Celsius).
Obviously, if the matter were in the expansion coefficients, the most unreliable contact would be between the steel and aluminum conductor, because their expansion coefficients differ by a factor of two.
But even without tabular data it is clear that the differences in linear thermal expansion are relatively easily compensated by the use of reliable clamps that create a constant pressure on the contact. Expand the metals, compressed, for example, with a well-tightened bolted joint, remains only to the side, and temperature changes can not seriously weaken the contact.
The option with the oxide film is also not quite true. After all, this same oxide film allows you to connect aluminum conductors with steel and with other aluminum conductors. Yes, of course, the use of a special lubricant against oxides is recommended, yes, a systematic revision of compounds involving aluminum is recommended. But all this is allowed and works for years.
But the version with a galvanic pair really has a right to exist. But here, after all, it does not do without oxides. After all, the copper conductor is also quickly covered with oxide, with the only difference that copper oxide conducts more or less current.
But if a copper and aluminum conductor are connected, their oxides have the possibility of dissociation, that is, decay into charged ions. Dissociation is possible due to natural moisture, which is always in the air. The ions of aluminum and copper oxides, being particles with different electrical potential, begin to take part in the process of current flow. A process known as "electrolysis" begins.
In the course of electrolysis ions transfer charges and move themselves. But, in addition, ions are after all metal particles of conductors. When they move, the metal collapses, shells and voids are formed. Especially it concerns aluminum. Well, where there are voids and shells, there is no longer any reliable electrical contact. Bad contact starts to get warm, gets worse and so on until the fire.
Note that the higher the humidity of the environment, the more intensively all the listed processes take place. A non-uniform thermal expansion and non-conductive layer of aluminum oxide are only aggravating factors, nothing more.